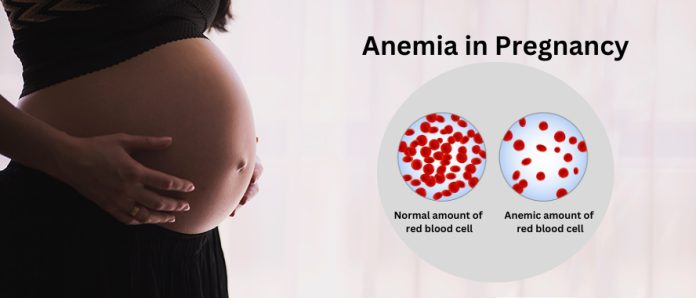
The number of pregnant women who have anaemia, which is the medical term for blood shortage, is very high in Nigeria.
According to the Chief Medical Director, Lagos University Teaching Hospital (LUTH), Prof. Lanre Adeyemo, anaemia in pregnant women increases the risk of maternal and neonatal mortality.
He spoke in Lagos at the IVON clinical trial.
 The CMD said, “Anaemia in pregnancy is highly prevalent in African countries. Globally, anaemia is the commonest medical condition affecting pregnant women and in Africa, about 50 per cent of all pregnant women are affected.
The CMD said, “Anaemia in pregnancy is highly prevalent in African countries. Globally, anaemia is the commonest medical condition affecting pregnant women and in Africa, about 50 per cent of all pregnant women are affected.
“In Nigeria, the most populous country in Africa, about three in five pregnant women have anaemia. The condition, which is mostly caused by iron deficiency, is associated with an increased risk of maternal and perinatal morbidity and mortality.
“It is recognised as a major global health problem with an indicator dedicated to tracking reduction efforts of anaemia in women 15–49 years of age, including pregnant women, Therefore, IVON clinical trial is a significant milestone in global efforts to reduce maternal and child morbidity and mortality, and the contributions of IVON trial team cannot be overemphasised.”

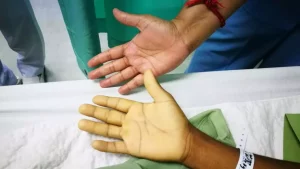
Adeyemo said the outcome of the IVON clinical trial, “will change clinical obstetrics practice in Nigeria and other developing nations of the world, and ultimately improve maternal and child health outcomes and help medical industrialisation.”
He said the ongoing efforts would mark a critical turning point in international efforts to lower rates of morbidity and mortality among mothers and children.
Adeyemo said, “The Federal Ministry of Health and Social Welfare under my leadership has developed a four-point agenda. The key vision behind the four-point agenda of the ministry is that of President Bola Ahmed Tinubu, GCFR.
“The agenda are: improving the quality of governance and leadership of hospitals; the regulatory capacity of agencies under the ministry; promoting medical industrialisation; and improving health security/investment in public health.”
“Seventy per cent of the nation’s pharmaceuticals, including drugs used in IVON trial, are imported from countries that are just like Nigeria; while Nigeria does not even produce its own vaccines. This narrative needs to change,” he added.
Also speaking at the event, the Director for Center Clinical Trials, Prof. Bosede Afolabi, said recent research had established that intravenous iron was better than oral iron for treating anaemia in pregnant women.
 “We found that the intravenous iron is safe and effective compared to oral iron. We are still publishing, so we won’t give away all our results.
“We found that the intravenous iron is safe and effective compared to oral iron. We are still publishing, so we won’t give away all our results.
“We are waiting for the paper to be published, but we found that it was effective in reducing iron deficiency anaemia in women, pregnant women.
Most of the time in pregnancy, more than 90 per cent of prescribers use just oral iron to treat anaemia. For now, the cost of the drugs is $40,000 but the drug is still in India.
“What we are trying to show is that one dose of intravenous is good, safe, and an effective alternative for women who might not be able to tolerate the oral iron. We are in the process of publishing.
“We have submitted the publication for this trial of our findings to a very reputable journal, and we are waiting to hear from them once it is published, then we will go to town to disseminate it,” Afolabi said.