Blood pressure medication from Glenmark Pharmaceuticals is being voluntarily recalled by the company over failed dissolution amid serious safety fears.
A total of 114 batches of Potassium Chloride Extended-Release 750mg Capsules, 100 count and 500 count are being recalled and consumers are advised to consult their physician before they stop using the product or if they experience any problems that could be related to it.
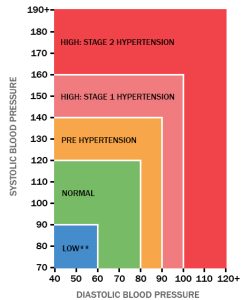
This warning was issued by the US-based Food and Drug Administration and is for America patients. The UK NHS does not use potassium chloride to treat heart conditions.
The company announced the recall over fears that the capsules won’t dissolve properly which could cause high levels of potassium. Potassium is often used to treat high blood pressure, yet extreme amounts of the vitamin can have devastating side-effects.
The announcement, shared by the FDA, noted that high potassium levels, also known as hyperkalemia, can result in irregular heartbeats that could lead to potentially cardiac arrest.
For those who use the capsules as a chronic medication, particularly those with underlying comorbidities or conditions like hypertension, heart failure or renal dysfunction, the company warned: “There is a reasonable probability of developing hyperkalemia that may lead to a range of severity of adverse events.”
 These “adverse events” include: “cardiac arrhythmias, severe muscle weakness, and death”. However, the notice highlighted that there have been no reports of hyperkalemia or “serious adverse events” related to the recall.
These “adverse events” include: “cardiac arrhythmias, severe muscle weakness, and death”. However, the notice highlighted that there have been no reports of hyperkalemia or “serious adverse events” related to the recall.
Glenmark Pharmaceuticals shared a list of the specific batch numbers and expiration dates of the bottles involved in the voluntary recall in a document circulated by the FDA. American Health Packaging voluntarily recalled 21 batches of the same medicine on behalf of BluePoint Laboratories.

The FDA has also urged people to report any adverse events to its MedWatch Adverse Event Reporting program via mail, fax or online.


