The Bill & Melinda Gates Medical Research Institute has announced that a Phase three clinical trial to assess the efficacy of the M72/AS01E tuberculosis vaccine candidate is now underway, with first doses given in South Africa, where TB takes a heavy toll.
The institute disclosed this in a press statement.
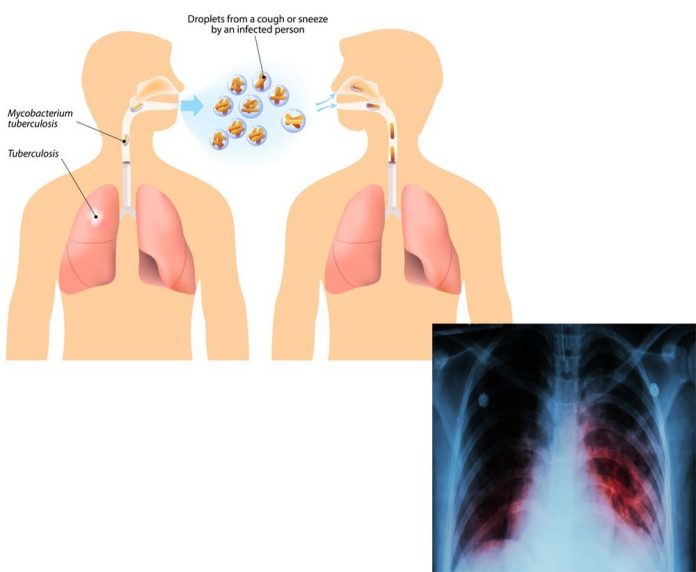
103-year-old BCG vaccine protects babies and young children against severe forms of TB, but it offers inadequate protection for adolescents and adults against the pulmonary form of the disease, which is primarily responsible for transmission of the TB bacterium
If shown to be well-tolerated and effective, M72/AS01E could potentially become the first vaccine to help prevent pulmonary TB in adolescents and adults, the most common form of the disease, and the first new TB vaccine in over a century.
According to the World Health Organisation, an estimated 10.6 million people fell ill with TB in 2022 and 1.3 million died — over 3,500 people per day.
The disease primarily affects people in low- and middle-income countries, and those at highest risk are often living in poverty, with poor living and working conditions and undernutrition. In South Africa alone, around 280,000 people are diagnosed with TB each year.
an estimated 10.6 million people fell ill with TB in 2022 and 1.3 million died — over 3,500 people daily
While TB is one of the world’s deadliest infectious diseases — and the leading cause of death among people living with HIV — the only available TB vaccine, BCG, dates back to 1921. It protects babies and young children against severe forms of TB, but it offers inadequate protection for adolescents and adults against the pulmonary form of the disease, which is primarily responsible for transmission of the TB bacterium.
“The launch of this pivotal Phase 3 trial demonstrates our commitment to harnessing the power of medical innovation to fight diseases like TB that are particularly devastating for low- and middle-income countries,” said Emilio A. Emini, Ph.D., CEO of the Gates MRI.
“Clinical study of the vaccine will still require years, but our incredible partners in South Africa and elsewhere who have come together for the Phase 3 study share our hope in the vaccine’s potential.”
TB is one of the world’s deadliest infectious diseases — and the leading cause of death among people living with HIV — the only available TB vaccine, BCG, dates back to 1921
The Gates MRI, a nonprofit organization and subsidiary of the Bill & Melinda Gates Foundation, is sponsoring the trial, which is supported by funding from the Gates Foundation and Wellcome.
The M72/AS01E vaccine candidate has been in development since the early 2000s. It was originally designed and clinically evaluated by the biopharma company GSK up to the proof-of-concept phase (Phase 2b), in partnership with Aeras and the International AIDS Vaccine Initiative (IAVI) and was funded by GSK and in part by the Gates Foundation.
At full capacity, the trial will include up to 20,000 participants, including people living with HIV, at up to 60 trial sites in seven countries — South Africa, Zambia, Malawi, Mozambique, Kenya, Indonesia and Vietnam.
TB is both a health problem and a socioeconomic problem. He said the disease primarily affects people during their prime working years, leaving families without income and children without parents
“After dedicating over 20 years to developing this essential candidate vaccine, we at GSK are delighted that the Phase 3 trial is underway. Developing and ensuring access to global health products is complex but our collaboration with the Gates MRI, Wellcome and the Gates Foundation exemplifies the transformative power of leveraging diverse partners’ expertise to change the trajectory of challenging diseases, like TB, which place a huge burden on communities around the world,” said Deborah Waterhouse, CEO, ViiV Healthcare and President, Global Health, GSK.
Alemnew Dagnew, M.D., who leads development of M72/AS01E at the Gates MRI said if effective, M72/AS01E could reinvigorate a global fight against TB that has been weakened by the COVID-19 pandemic.
Dagnew noted that TB is both a health problem and a socioeconomic problem. He said the disease primarily affects people during their prime working years, leaving families without income and children without parents. Almost half of TB-affected households face costs higher than 20 per cent of their household income.
Almost half of TB-affected households face costs higher than 20 per cent of their household income
“We must continue to move with urgency to develop and equitably deliver innovative tools with the potential to transform the prevention, diagnosis, and treatment of TB,” said Trevor Mundel, President, Global Health, Bill & Melinda Gates Foundation.
It is anticipated that it will take up to five years to complete the trial, followed by data analysis and then preparation for submission of data to regulatory authorities.