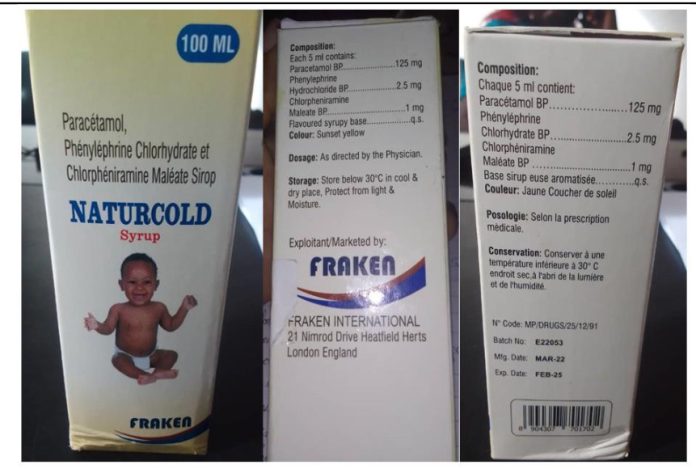
The World Health Organisation said a batch of cold syrup sold in Cameroon under the brand name Naturcold contained extremely high levels of contaminated toxic ingredients.
The National Agency for Food and Drugs Administration and Control had in April warned Nigerians against the use of Naturcold, believed to have caused the deaths of six children under the age of five at a health facility in the health district of Fundong, in the North-West region of Cameroon.
In 2022, more than 300 children – mainly aged under five – in Gambia, Indonesia, and Uzbekistan died of acute kidney injury, in deaths associated with cough syrups for children with confirmed or suspected contamination with high levels of diethylene glycol and ethylene glycol.
WHO said Naturcold Syrup sold in Cameroon was first reported on March 13, 2023, and samples of the syrup were made available to WHO on June 27, 2023.
It said the analysis of the syrup found that the product contained unacceptable amounts of diethylene glycol as contaminants.
“Diethylene glycol was detected in samples of Naturcold as much as 28.6 per cent. The acceptable limit for Diethylene Glycol is no more than 0.10 per cent,” WHO said.
Diethylene glycol and ethylene glycol are toxic to humans when consumed and can prove fatal.
The global health body said the substandard products are unsafe, and their use, especially in children, may result in serious injury or death.
“Toxic effects can include abdominal pain, vomiting, diarrhoea, inability to pass urine, headache, altered mental state and acute kidney injury which may lead to death,” it added.
It said the stated manufacturer of the affected product is listed on the product packaging as Fraken International (England) but the United Kingdom national regulatory authority, the MHRA, confirmed that no such company exists in the UK.
“Enquiries are still underway to determine the origin of the product. Therefore, the stated manufacturer has not provided guarantees to WHO on the safety and quality of these products.
“The product referenced in this Alert may have marketing authorisations in other countries or regions. It may also have been distributed through informal markets to neighbouring countries,” it added.