The National Agency for Food and Drug Administration Control has banned the sale of Dex luxury bar soap because it contains Butyphenyl Methylpropional.
Scientists describe Butylphenyl Methylpropional as a common fragrance ingredient that has a nice floral scent and also goes by the name Lilial.
“It is a known fragrance allergen and, as of 1st of March 2022, it has been banned in the European Union due to animal studies showing a possible link to infertility in rats,” says INCIDecoder, a body that decodes skincare ingredients with science-based but easy-to-understand explanations.
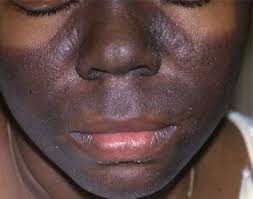
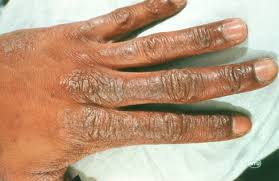
According to various online sources, Butylphenyl Methylpropional (aka Lilial), which functions as a fragrance and masking ingredient, is connected with possible bioaccumulation, organ system toxicity, endocrine disruption and allergies, and contact dermatitis.
Cosmetics containing perfume allergen lilial are no longer allowed to be sold in the United Kingdom. The ‘making available on the market’ deadline for products containing the fragrance ingredient was 15 December 2022.
Based on its evaluation, an International Fragrance Association (IFRA) Standard was established in 2020 (Amendment 49) in the United States of America, prohibiting the use of butylphenyl methylpropional in finished product application with oral and lips exposure, and restricting its use in other cosmetics products.
Its safety is still controversial, as it can be sensitizing, skin irritant and a potential allergen, according to the EU-based Scientific Committee on Consumer Safety [SCCS).
Another downside it that it also seen as an endocrine disruptor and even possibly carcinogenic, says the SCCS.
butylphenyl methylpropional is also seen as an endocrine disruptor and possibly carcinogenic
According to a statement posted on NAFDAC’s official handle on Thursday, the product, Dex Luxury Bar Soap (No 6 Mystic Flower), manufactured in Turkey, contains BMHCA, a substance prohibited in cosmetic products due to its potential harm to the reproductive system and skin sensitization.
The post reads: “Public Alert No. 012/2024.Ban on the sale of Dex Luxury Bar Soap due to Butyphenyl Methylpropional (BMHCA) content.
“NAFDAC is notifying the public of the ban on the sale of Dex Luxury Bar Soap (No 6 mystic flower) by the European Union (EU).
“The product does not comply with the Cosmetic Products Regulation as it is said to contain Butyphenyl Methylpropional (BMHCA) which is prohibited in cosmetic products due to its risk of harming the reproductive system, causing harm to the health of the unborn child, and may cause skin sensitization.
“As a result, a ban on the marketing of the product has been placed by some regulatory and public authorities in the EU.
“Product Name: Dex Luxury Bar Soap (No 6 Mystic Flower), Product Brand: DEX, Country of Manufacture: Turkey, Model: 11.11.22, Barcode: 8694965531, Category: Cosmetics.
“Although this product is not on the NAFDAC database, importers, distributors, retailers, and consumers are advised to exercise caution and vigilance within the supply chain to avoid the importation, distribution, sale, and use of the above-mentioned product.
“Healthcare professionals and consumers are advised to report any suspicion of adverse reactions or substandard and falsified regulated products to the nearest NAFDAC office.”


